Abstract
Adoptive transfer of cytolitic Natural Killer (NK) cells is a promising immunotherapeutic modality for hematologic and other malignancies. However, limited NK cell in vivo persistence and proliferation have been challenging clinical success of this therapeutic modality. Here we present a reliable, scalable and GMP-compliant culture method for the expansion of highly functional donor NK cells for clinical use.
Nicotinamide (NAM), a form of vitamin B-3, serves as a precursor of nicotinamide adenine dinucleotide (NAD) and is a potent inhibitor of enzymes that require NAD including ADP ribosyltransferases and cyclic ADP ribose/NADase. As such, NAM is implicated in the regulation of cell adhesion, polarity, migration, proliferation, and differentiation.
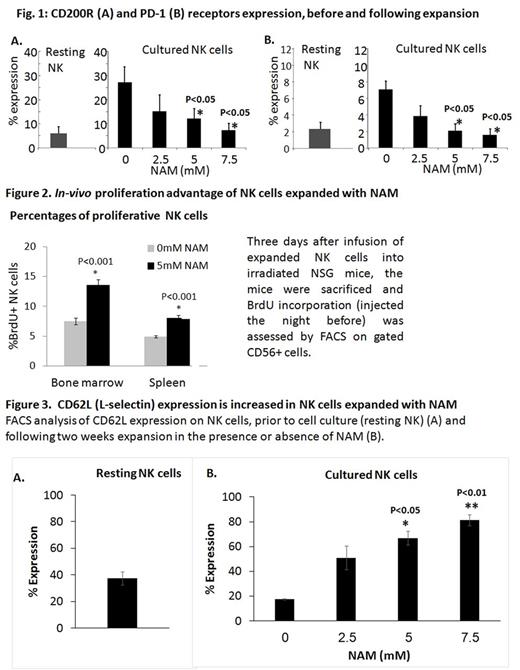
We have previously reported that NAM augments tumor cytotoxicity and cytokine (TNFα and IFN-γ) secretion of NK cells expanded in feeder-free culture conditions stimulated with IL-2 or IL-15. Immunophenotype studies demonstrated NK cells expanded with NAM underwent typical changes observed with cytokine only-induced NK cell activation with no significant differences in the expression of activating and inhibitory receptors. CD200R and PD-1 receptors were expressed at low levels in resting NK cells, but their expression was up-regulated following activation in typical cytokine expansion cultures. Interestingly, the increase in CD200R and PD-1 was reduced by NAM, suggesting these NK cells to be less susceptible to cancer immunoevasion mechanisms (Fig 1).
In vivo retention and proliferation is a pre-requisite for the success of NK therapy. We have reported that NK expanded with NAM displayed substantially better retention in the bone marrow, spleen and peripheral blood of irradiated NSG mice. Using a carboxyfluorescein succinimidyl ester (CFSE) dilution assay, we demonstrated increased in vivo proliferation of NAM-cultured NK cells compared with cells cultured without NAM. These results were recently confirmed using a BrdU incorporation assay in irradiated NSG mice (Fig.2). These findings were mechanistically supported by a substantial increase in CD62L (L-selectin) expression in cultures treated with NAM. CD62L is pivotal for NK cell trafficking and homeostatic proliferation and its expression is down regulated in IL-2 or IL-15 stimulated cultures (Fig. 3).
These data provided the foundation for the development of a feeder cell-free scalable culture method for clinical therapy using apheresis units obtained from healthy volunteers. CD3+ cells were depleted using a CliniMACS T cell depletion set. Following depletion, the CD3- fraction was analyzed for phenotypic markers and cultured in closed-system flasks (G-Rex100 MCS, Wilson Wolf) supplemented with 20ng/ml IL-15 or 50ng/ml IL-2 GMP, 10% human serum, minimum essential medium-α and NAM USP for two weeks. While at seeding, NK cells comprised 5-20% of total culture seeded cells, at harvest, NK cells comprised more than 97% of the culture. Although overall contamination of the NK cultures was low with either IL-15 or IL-2, a lower fraction of CD3+ and CD19+ cells was observed with IL-15 vs IL-2 (0.2±0.1% vs. 0.4±0.2% and 1.3±0.4% vs. 2.4±0.6%, respectively). Consequently, we decided to use IL-15 for clinical manufacturing. Optimization of NAM concentration studies showed similar expansion with 2.5 and 5 mM and a decrease in expansion with 7.5 mM NAM. Since NAM at 5 mM had a stronger impact on CD62L expression and on the release of IFNγ and TNFα than NAM at 2.5 mM, we selected 5mM NAM for clinical manufacturing.
Overall median NK expansion after two weeks in closed G-Rex flasks supplemented with IL-15 and 5mM NAM was 50-fold (range 37-87). An additional and significant increase in expansion was obtained after doubling the culture medium one week post seeding. While there was a marked advantage for single culture feeding, more feedings had less impact on NK expansion and had a negative effect on the in vivo retention potential. Our optimized expansion protocol therefore involved one feeding during the two weeks expansion duration resulting in 162±30.7-fold expansion of NK cells relative to their input number in culture.
Based on these data, we have initiated a clinical trial at University of Minnesota, to test the safety and efficacy of escalating doses (2 x 107/kg - 2 x 108/kg) of our novel NAM NK cell product in patients with refractory non-Hodgkins lymphoma and multiple myeloma (NCT03019666).
Peled: Gamida Cell: Employment, Equity Ownership. Brachya: Gamida Cell: Employment. Persi: Gamida Cell: Employment. Lador: gamida Cell: Employment, Equity Ownership. Olesinski: gamida cell: Employment. Landau: gamida cell: Employment, Equity Ownership. Galamidi: gamida cell: Employment. Peled: Biokine: Consultancy; Biosight: Consultancy. Miller: Celegene: Consultancy; Oxis Biotech: Consultancy; Fate Therapeutics: Consultancy, Research Funding. Bachanova: Oxis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Zymogen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Seattle-Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis Pharmaceuticals Corporation: Membership on an entity's Board of Directors or advisory committees, Research Funding; Juno: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal